Well as the title mentions, we were able to get the test in amongst our early November snow days. However, with the snow days there weren't many days to prepare for the exam in class. With this in mind, I decided to send out to everyone directions for a practice exam that they could do outside of class on-line. We reviewed the answers in class with the time we had and were able to get through all the review. I also heard this week that some other classes have moved towards online assignment work. As I prepare for next trimester and transition into Chemistry B I will be continuing with integrating technology into the classroom. Glad to see this trend is catching on.
Sunday, November 23, 2014
Sunday, November 16, 2014
Week 11 - Demos
This week we continued balancing equations, writing formulas, and identifying different types of reactions. Then capped it off with demos!!!!
The preparation:
elephant toothpaste, pumpkin style
the decomposition of hydrogen peroxide
copper metal reactants with nitric acid
The preparation:
elephant toothpaste, pumpkin style
the decomposition of hydrogen peroxide
Next week includes last test and exam review!
Sunday, November 9, 2014
Week 10 - Naming , Formula Writing, More Naming, and Balancing!
So what is the difference between sodium and the sodium ion? Would you believe one electron? However, one is highly reactive in water and must be stored under oil and the other is simply combined with a majority of nonmetals and then dissolves readily in water. So the idea of naming things correctly is pretty important. Whether its sulfur trioxide (SO3) vs sulfite (SO3 2-) again it is important to understand the naming of substances. With naming also comes writing formulas correctly. This includes writing formulas of acids, formulas of molecules, and formulas of salts. Once we progressed from naming and writing formulas we moved to balancing equations. So this week and next will be lots and lots of practice. Exams are coming soon! Study guides will be arriving soon.
Finally, we ended the week with identifying the five types of reactions.
Finally, we ended the week with identifying the five types of reactions.
Sunday, November 2, 2014
results are in #molympics press release
Molympics 2014 Press Release
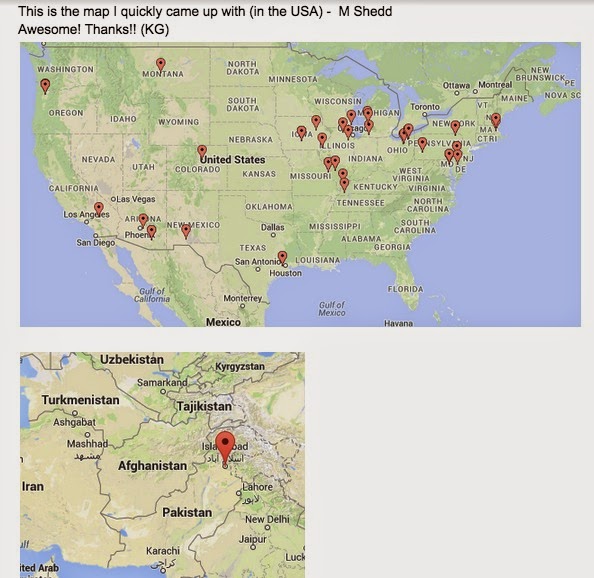
On October 23, 2014 chemistry students at high schools in Tucson AZ, Indianola IA, Highland IL, Burlington MA, Hudsonville MI, St. Louis MO, Las Cruces NM, Chesterland OH, Medina OH, Newberg OR, Exton PA, Harrison City PA, Montrose PA, and the Beaconhouse Margalla Boys school in Islamabad Pakistan competed in the “Mole Olympics” (Molympics). Why? To learn about Avogadro’s number, 6.02 x 1023 or one mole, and its importance in chemistry. The mole is a counting unit in chemistry, and represents the number of atoms in 12.0115 grams of carbon. Mole Day is celebrated from 6:02 am to 6:02 pm on 10/23 each year.
Students honed their skills at estimation, measurement, dimensional analysis and problem solving by competing in the Tally Mark Challenge, the Sponge Squeeze, the Mole of Metals, the Stopper Tower and the Avogadro Fitness Challenge events. At stake were bragging rights and a trophy that will travel to the winning school each year.
This year’s top school is Burlington High School in Burlington, MA led by chemistry teacher Ms. Wendy Czerwinski. Two schools tied for second place. Highland High School in Medina, OH led by chemistry teacher Mr. Chris Luker and Tanque Verde High School in Tucson, AZ led by chemistry teacher Ms. Grazyna Zreda. In third place was Mayfield High School in Las Cruces, NM led by chemistry teacher Ms. Ann Gardner. Fourth place went to West Geauga High School in Chesterland, OH led by chemistry teacher Ms. Kristin Gregory. Hudsonville High School in Hudsonville, MI led by chemistry teacher Mr. Doug Ragan tied for fifth place with CS Lewis Academy of Newberg, OR led by chemistry teacher Ms. Pam Chambers.
The Molympics competition, now in its second year, is the result of a cross-country collaboration between five high school chemistry teachers who met and planned the event via Twitter. Teachers Wendy Czerwinski of Burlington MA, Ann Gardner of Las Cruces NM, Kristin Gregory of Chesterland OH, LuAnn Lee of Newberg OR, and Doug Ragan of Hudsonville MI also used Google apps for education to organize and run the event and to spread the word to participants worldwide. Based on the success of year two, next year’s Molympics should be bigger and better than ever.
Molympics Planning Team at the Biennial Conference on Chemical Education 2014
Kristin Gregory, Doug Ragan, Wendy Czerwinski Not pictured: Ann Gardner, LuAnn Lee
Thanks to Kristin Gregory for this!
It was a great learning experience, I got a great heads up on what we need to focus on regarding dimensional analysis and the time it will require. I was quite pleased with the results and excited to see #molympics grow. Week 9 is done and with week 10 we will be continuing to name compounds, write formulas, and then balance equations. Exams are coming soon!!!
-Mr. Ragan
Sunday, October 26, 2014
#Molympics, enough said!
Well, it was a great week. I had a total of 40 parent teacher conferences Monday night and another 26 Wednesday night for a grand total of 66 conferences. I would like to say thank you to all of the parents that came out and I am so glad I got to meet you.
Next we had the late start on Wednesday followed by the test on Electron Configuration and the Periodic Table. I was overall pleased with the results of the test and reminder any make up works needs to be completed within the next two weeks before exams and exam review begin. Reminder the element project is due this Tuesday and I look forward to creating the Periodic Table on the ceiling.
Once the test was complete, we finished out Thursday celebrating Mol Day http://www.moleday.org/ and then participated Thursday and Friday in Molympics. We competed against somewhere close to 19 other schools across the U.S. including a school in Pakistan doing several activities that related to the mol. We should find out later this week what school won the awesome trophy.
Next we had the late start on Wednesday followed by the test on Electron Configuration and the Periodic Table. I was overall pleased with the results of the test and reminder any make up works needs to be completed within the next two weeks before exams and exam review begin. Reminder the element project is due this Tuesday and I look forward to creating the Periodic Table on the ceiling.
Once the test was complete, we finished out Thursday celebrating Mol Day http://www.moleday.org/ and then participated Thursday and Friday in Molympics. We competed against somewhere close to 19 other schools across the U.S. including a school in Pakistan doing several activities that related to the mol. We should find out later this week what school won the awesome trophy.
Sunday, October 19, 2014
Periodic Table and the Element Ball Project
This week we finished up with the chapter on electron configuration and began our next chapter on the Periodic Table. We jumped into lab to begin organizing elements then began learning about about the trends in reactivity.
We have lots of pieces to start putting together as we begin sequencing valence electrons to charges to formulas to naming to chemical reactions. Things will be busy.
Plans next week include a test on Wednesday and to continue gathering facts on our element ball project.
Element Ball Project
Every student was assigned an element in which they need to gather 20 facts. As the element balls come in (Oct 28) then we will construct a 3D periodic table on the ceiling and discuss some of the interesting facts about the elements. Next week includes Parent teacher conferences on Monday and Wednesday night, test on Wednesday (late start), then #molympics on Thursday and Friday. Its going to be a great week. Also be sure to check out my website at http://dragan38.wix.com/mrragan for more course information.
Saturday, October 11, 2014
Week 6: Atomic Models and Diagonal Rules
This week we continued with our look at atomic models and began to focus more on the address system for electrons. And although we can't pinpoint exactly where the electron might be, I think I got it across to my students that it was similar to looking in the stands at the football game and having a pretty good idea that your parents or friends are up there somewhere on the home side, in that section, in that row and maybe even in those seats! But their changing seats like musical chairs and the music never stops playing!
Next it sank in that we were half through the trimester and I was excited to hear students recommending to other students to watch the videos because the video really explained things well. We started into the address system and I began spouting out 1s^2 2s^2 2p^6 alright, alright, wait what? As we continued I began seeing more and more light bulbs going off of understanding and I'll know more next week with assessments. In the meantime we took Friday to get a grip on the so called diagonal building of lowest energy to highest energy and took time out to understand the so called diagonal rule with the diagonal rule challenge. The object, feed an extension cord through the order of the diagonal rule from 1s^2 to 7p^6 as quickly as possible!
Friday, October 3, 2014
Week 5 and Things Went Nuclear
This week we finished up atomic structure and calculated all the components of the atom. We determined the number of protons, electrons, neutrons, mass numbers, atomic mass, charges, etc. Watched a video on carbon-14 dating and discussed isotopes. We then used the iPads then to test our knowledge of these concepts.
The wonderful thing about this program is that
it is web based so for those students that wanted
to continue to practice they had the capability to
do so using their own device such
as a home computer, iPad, or smart phone.

Overall, several students were quite successful on their quiz as we wrapped up the unit on atomic structure.
Next, so many advances in what we know about the atom resulted from the work of nuclear chemistry. We continued with a newer model of the atom and looked at nuclear energy, the harmfulness of radon gas, and discussed nuclear power plants. We continued with several calculations of radioactive decay and how nuclear atoms can undergo transmutation. I used the app classkick to allow the students to work some problems in class on the iPads and then provide me with the data I needed to quickly assess. Overall I was pleased with the results.
Next week we continue with the harmful effects of radiation and prepare for our test on Tuesday.
The wonderful thing about this program is that
it is web based so for those students that wanted
to continue to practice they had the capability to
do so using their own device such
as a home computer, iPad, or smart phone.

Overall, several students were quite successful on their quiz as we wrapped up the unit on atomic structure.
Next, so many advances in what we know about the atom resulted from the work of nuclear chemistry. We continued with a newer model of the atom and looked at nuclear energy, the harmfulness of radon gas, and discussed nuclear power plants. We continued with several calculations of radioactive decay and how nuclear atoms can undergo transmutation. I used the app classkick to allow the students to work some problems in class on the iPads and then provide me with the data I needed to quickly assess. Overall I was pleased with the results.
Next week we continue with the harmful effects of radiation and prepare for our test on Tuesday.
Tuesday, September 30, 2014
Oct 1st is Flipped Day 2014
Just wanted to inform you that a handout is coming home regrading my flipped classroom. Oct 1st is Flipped Day and just wanted to share a quick link from NPR regarding the flipped classroom.
http://www.npr.org/2012/12/07/166748835/more-teachers-flipping-the-school-day-upside-down
Any questions feel free to contact me.
http://www.npr.org/2012/12/07/166748835/more-teachers-flipping-the-school-day-upside-down
Any questions feel free to contact me.
Saturday, September 27, 2014
Week 4 & were Modeling
Week 4 and this past Tuesday was our first test. Class average was an 82 and possibly a little higher once we cleared up some errors with how some of the students read some questions. Once that was complete we began our next unit with atomic structure and the models of the atom. Starting with the ancient Greeks and then progressing up to what we know about the current size of the atom. This week included a couple more videos and we learned that if we enlarged an atom to the size of an football stadium then the nucleus would be the size of a marble placed on the fifty yard line. The rest empty space!
http://sun.menloschool.org/~dspence/chemistry/atomic/images/nucleus_marble
With that we decided to try our hand at being able to picture something without actually being able to see it. Similar to how scientists determined what the atom looked like without being able to see it. So we all took part in: Rutherford's Gold Foil pizza box prediction activity.
http://sun.menloschool.org/~dspence/chemistry/atomic/images/nucleus_marble
With that we decided to try our hand at being able to picture something without actually being able to see it. Similar to how scientists determined what the atom looked like without being able to see it. So we all took part in: Rutherford's Gold Foil pizza box prediction activity.
Who knew rolling a marble in a pizza box could be so much fun.
Next week we start off Monday with some atomic structure practice using the our technology for our quiz Tuesday.
Then we start NUCLEAR!
Sunday, September 21, 2014
Week 3 and We Flipped for Homecoming!
Another great week in chemistry for week 3 Homecoming Week and this brought many new things including the introduction of Chapter 3: Scientific Measurement. With that I introduced the students to what has been referred to as flipped learning http://jonbergmann.com/what-is-the-flipped-class/#comment-182. I have been flipping my classroom now for over two years and have totally enjoyed all the benefits it has to offer. I went as far as set up a green screen at home which has allowed me to place myself more into the videos. I can't wait to interact more with my backgrounds as I explore all that the green screen capabilities have to offer.
The initial feedback I got from students was positive regarding the videos and I hope to gain more time for one on one instruction with each of the students in the next few weeks.
Amongst being comfy, hat days, dressing in all black, and showing eagle pride we saw that 10lb bowling balls do indeed float in water as we calculated density and we determined that Indiana Jones had no chance in replacing the gold idol in the movie "Raiders of the Lost Ark".
It really was a busy week as we prepare for our first test on Tuesday and ended in great style with an awesome homecoming assembly!
Mr. Ragan
The initial feedback I got from students was positive regarding the videos and I hope to gain more time for one on one instruction with each of the students in the next few weeks.
 |
| Green screen |
Amongst being comfy, hat days, dressing in all black, and showing eagle pride we saw that 10lb bowling balls do indeed float in water as we calculated density and we determined that Indiana Jones had no chance in replacing the gold idol in the movie "Raiders of the Lost Ark".
Also this week, i got my iPads back from being reset and we had the chance to use them for assessment practices and in preparation for the quiz on Friday.
It really was a busy week as we prepare for our first test on Tuesday and ended in great style with an awesome homecoming assembly!
Mr. Ragan
Saturday, September 13, 2014
Week 2
This past week in Chem A we continued classifying matter and everything from elements to macaroni salad to rocky road ice cream. We saw how elements can be considered the building blocks of the building blocks of all matter.
All students successfully passed the safety quiz earlier in the week and in no time we were in the lab looking at how what we see as physical and chemical reactions can also be viewed with atomic level representations. I got to break out 1970's camera and describe to students the chemistry of flashbulbs! Remember those!
Next week is going to be busy as we continue in all things measurement and how important that is in lab!
All students successfully passed the safety quiz earlier in the week and in no time we were in the lab looking at how what we see as physical and chemical reactions can also be viewed with atomic level representations. I got to break out 1970's camera and describe to students the chemistry of flashbulbs! Remember those!
Next week is going to be busy as we continue in all things measurement and how important that is in lab!
Thursday, September 11, 2014
Materials needed for class:
Required Class Materials: CHEM A, B & Conceptual Chemistry.
1. 3 ring binder with your name on it, loose-leaf paper is provided
2. Pen and pencil
3. Textbook can be checked out of the library and a class set is provided.
4. 3x5 (small) index cards – (for element and ion flash cards)
5. Scientific calculator – it does not need to be a graphing calculator.
2. Pen and pencil
3. Textbook can be checked out of the library and a class set is provided.
4. 3x5 (small) index cards – (for element and ion flash cards)
5. Scientific calculator – it does not need to be a graphing calculator.
About me
A little bit about me....
Born and raised in Newport News, Virginia. I Graduated from Denbigh High School in 1990. Attended Longwood College now University and obtained my BS in Chemistry with my secondary teacher certification in 1994. Taught Chemistry, Pre IB Chemistry, and AP Chemistry at my old high school for 6 years. Obtained my MS in Chemical Education from Purdue University in 2004.
Completed the Target Inquiry Program for chemistry teachers at GVSU to advance my degree. Happily married to Dr. Deborah Herrington who is a chemistry professor at GVSU and have two awesome kids, Lexie age 8 and Tyler age 6.
FYI:
Started playing saxophone in the 6th grade
Was drum major my junior and senior year of high school
Marched as Drum Major in the Inaugural Parade for the Governor Of Virginia
Was assistant marching band director for 6 years, while teaching in VA.
Won the Tidewater Alliance of Chemistry Teachers Teacher of the Year award
Have given talks at the local and national level including chemical demonstration shows at the State level.
Served on the Purdue racquetball traveling team
Won the GVSU racquetball intramural championship
Born and raised in Newport News, Virginia. I Graduated from Denbigh High School in 1990. Attended Longwood College now University and obtained my BS in Chemistry with my secondary teacher certification in 1994. Taught Chemistry, Pre IB Chemistry, and AP Chemistry at my old high school for 6 years. Obtained my MS in Chemical Education from Purdue University in 2004.
Completed the Target Inquiry Program for chemistry teachers at GVSU to advance my degree. Happily married to Dr. Deborah Herrington who is a chemistry professor at GVSU and have two awesome kids, Lexie age 8 and Tyler age 6.
FYI:
Started playing saxophone in the 6th grade
Was drum major my junior and senior year of high school
Marched as Drum Major in the Inaugural Parade for the Governor Of Virginia
Was assistant marching band director for 6 years, while teaching in VA.
Won the Tidewater Alliance of Chemistry Teachers Teacher of the Year award
Have given talks at the local and national level including chemical demonstration shows at the State level.
Served on the Purdue racquetball traveling team
Won the GVSU racquetball intramural championship
Ryan Newman & Nascar fan
Was a visiting professor at GVSU.
Served as NHS co-advisor at HHS.
Currently a coach for HHS Science Olympiad Team
Started teaching Chemistry at Hudsonville in 2005 and love it!
Began teaching with a class set of iPads in 2010.
Totally love my iPad.
Began Flipped Classroom in Chemistry B 3rd trimester 2012
Was a visiting professor at GVSU.
Served as NHS co-advisor at HHS.
Currently a coach for HHS Science Olympiad Team
Started teaching Chemistry at Hudsonville in 2005 and love it!
Began teaching with a class set of iPads in 2010.
Totally love my iPad.
Began Flipped Classroom in Chemistry B 3rd trimester 2012
WIll be flipping Chemistry A in Fall 2013
Began sharing 30 iPad2 devices with Mrs. Webster in Dec. 2012
email me at: dragan@hpseagles.net
follow me @dragan39
7/2014
follow me @dragan39
7/2014
Sunday, September 7, 2014
One Word
Last year, we as a faculty at Hudsonville were given the task to create one word http://getoneword.com/ that would define our year. My word was "passion" and after four conferences this summer, I truly feel I embraced this word and so you can say I have a passion for chemistry and learning about chemistry and sharing all things related to chemistry. Which leads me to my new word "collaboration" for this year. I love collaborating with others to learn what's new, what's better and yet also share my knowledge so I can do the same for them.
This past week I introduced my students to the build a boat challenge. I had gotten this activity from a fellow chemistry teacher friend and decided to use it to build a culture of collaboration amongst my students. The results were incredible. The students were given a list of materials and 15 minutes for design and then 15 minutes to actually build the boat. The goal was to determine the best ratio between cost and the boats capability to float as many pennies as possible. What I witnessed was newly formed lab groups brainstorming, making designs and decisions, experimenting and most of all talking and collaborating. Of course this had turned into a competition with myself and fellow teachers from other parts of the U.S. again for the purpose of collaborating on what worked and what didn't.
Well it was great start and I really hope to refer back to my one word of collaboration for the remainder of the year. In my eyes, everyone was a winner, all the boats floated, everyone decided they could build a better build having seen everyone else's design and well having learned from one another in class. Isn't that the culture we want to establish? My students all agreed! Great 1st week!
Subscribe to:
Posts (Atom)